IDCRC Newsletter: February 2024
IDCRC Investigator Profile: Lisa Jackson, MD, MPH

Lisa Jackson, MD, MPH, is an infectious disease epidemiologist, clinical trialist, and senior investigator at the Kaiser Permanente Washington Health Research Institute (KPWHRI), and the principal investigator (PI) of KPWHRI’s Vaccine and Treatment Evaluation Unit (VTEU).
Spotlight: University of Rochester Site Visit
The IDCRC Leadership Group (LG) continues their Vaccine and Treatment Evaluation Unit (VTEU) site visits. On December 18, 2023, the IDCRC PIs, David Stephens, MD, Kathy Neuzil, MD, and Clinical Operations Unit (COU) member, Valeria Cantos, MD, were hosted by Ann Falsey, MD, and Angela Branche, MD, of the University of Rochester (UR) VTEU.
“The UR VTEU team was thrilled to host IDCRC leadership in December and highlight some of the strengths of the University of Rochester scientific community. Kathy, David, and Valeria interacted with our amazing clinical research team and had lunch with junior investigators and emerging scientists who have led recent studies or were recipients of UR VTEU pilot awards. IDCRC mentorship engaged with basic scientists around the cutting-edge immunologic and omics works related to respiratory viruses we are known for at the University of Rochester. We look forward to how these productive and interesting conversations will allow us to continue to foster scientific collaborations within the network,” says Branche.
The Leadership Group thanks the Rochester VTEU again for their hospitality and looks forward to visiting again soon.
Annual Awards Nominations
Deadline extended! Annual IDCRC Awards
nominations are due Friday, March 8.
The IDCRC presents annual awards to acknowledge the work of VTEU members who have made exceptional contributions to the IDCRC and VTEU missions. We are now taking nominations for 2024. Nominations can be made by any IDCRC LG or VTEU member. Nominators may submit a nomination for each award category.
All nominations must be received by Friday, March 8, 2024.
Award Nomination Categories:
- Investigator of the Year: A well-established VTEU investigator who has made exceptional contributions to research as it relates to the VTEU and IDCRC missions
- Early Career Investigator: A recent graduate or current participant of the IDCRC Mentoring Program who has made exceptional contributions to research as it relates to the VTEU and IDCRC missions.
- Staff STAR (Stellar Team Member Achieving Results) Award: A VTEU coordinator or administrator whose exceptional contribution has advanced and supported the quality of IDCRC supported research.
- Best Scientific Publication of the Year: An IDCRC cited publication in the last year that is considered to have had a high impact on the scientific community and/or has led to policy change.
- Leadership Group Award: An IDCRC member who has demonstrated exemplary leadership in their commitment to the IDCRC LG aims
Awards will be presented at the IDCRC Reception during the 2024 IDCRC Annual Meeting. Previous award recipients may be viewed on the Annual IDCRC Awards webpage.
Questions? Please contact Jacquelyn Manduley.
Events
Reminder: Abstracts for the 2024 Annual Conference on Vaccinology Research due today, February 29
The National Foundation for Infectious Diseases (NFID) invites abstract submissions of original research in the field of vaccinology through today, February 29. Abstracts are peer-reviewed for quality of research, educational, and scientific content. Accepted abstracts will be scheduled as either poster or oral presentations at 2024 ACVR.
Abstract submission topics include:
- Combatting Antimicrobial Resistance
- Epidemiology and Burden of Diseases
- Implementation Challenges and Solutions
- Innovation in Immunization
- Vaccine Disparities and Health Equity
- Vaccine Policy and Regulatory Pathways
- Vaccine Research, Development, Production, and Delivery
- Vaccine Safety, Monitoring, and Evaluation
- Vaccines Against Emerging and Re-Emerging Infectious Diseases
- Vaccines for Special Populations (Maternal Immunization, Immunocompromised, and High-Risk Groups)
Save the Date: 2024 IDCRC Annual Meeting
Save the date! This year’s annual meeting will take place from May 1-2, 2024. We look forward to seeing you there.
Date: May 1-2, 2024
Location: Hybrid - In-person and Virtual
5601 Fishers Lane,
Rockville, MD, 20892
More details to follow!
VTEU Highlight
Vanderbilt University Medical Center
Highlights from the most recent grant year were presented by all VTEUs at our 2023 Annual Meeting. This month we are featuring Vanderbilt University Medical Center. Note, these were created by the IDCRC Leadership Operations Center and is not intended to be a comprehensive list.
Publications
NOTE: Please include the following citation in any publications resulting from direct or indirect IDCRC support:
"Supported by the Infectious Diseases Clinical Research Consortium through the National Institute for Allergy and Infectious Diseases of the National Institutes of Health, under award number UM1AI148684. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health."
News
COVID-19 Vaccination and Boosting During Pregnancy Protects Infants for Six Months
Earlier results from the Multisite Observational Maternal and Infant COVID-19 Vaccine (MOMI-Vax) study revealed that when pregnant volunteers received both doses of an mRNA COVID-19 vaccine, antibodies induced by the vaccine could be found in their newborns’ cord blood.
This suggested that the infants likely had some protection against COVID-19 when they were still too young to receive a vaccine. Researchers at the NIAID-funded IDCRC, which conducted the study, did not know how long these antibody levels would last or how well the infants would actually be protected. The research team hoped to gather this information by following the infants through their first six months of life.
Flu and COVID cases are rising.
Here's what you can do to protect yourself.
Flu and COVID-19 infections have been increasing for weeks across the U.S., with high levels of flu-like illness reported in 31 states, including Georgia just before Christmas. New York City last week instituted a mask mandate for the city’s 11 public hospitals and similar measures were ordered at some hospitals in Los Angeles and Massachusetts. Health officials predict infections will continue to grow. Dr. Carlos del Rio, Distinguished Professor of Medicine in the Division of Infectious Diseases at Emory University School of Medicine speaks with GPB’s Peter Biello on these concerns.
Paxlovid can lessen the chance of a severe COVID-19 illness.
Why is it underused?
Tens of thousands of Americans are hospitalized with COVID-19 every week. Thousands die from it every month. And yet, an antiviral treatment proven to lessen the chances of severe outcomes is going underused. The drug, Paxlovid, is lauded by experts as a powerful tool that can prevent hospitalization and death from COVID-19. But the high price and doctors’ hesitation to prescribe the pills mean the five-day treatment isn’t getting to everyone who would benefit from it.
Another factor hurting uptake appears to be the long list of medications that shouldn’t be taken with Paxlovid. Many doctors may simply decide the risk of drug interactions isn’t worth it. “If people are on four or five different medications, it does tend to be a pain to double check ‘is there an interaction here?’” said Dr. Sarah George, an infectious diseases professor at St. Louis University. Seeing a possible significant drug interaction “tends to put a physician off from prescribing a drug, even if there is a workaround,” she said.
UMD doctor unpacks the growing concern over measles outbreak, vaccination gaps
With the recent increase in measles cases in the United States, FOX45 News interviewed Dr. Mathew Laurens, a pediatric infectious disease specialist with the University of Maryland School of Medicine, about the seriousness of the disease and the importance of vaccines.
Training
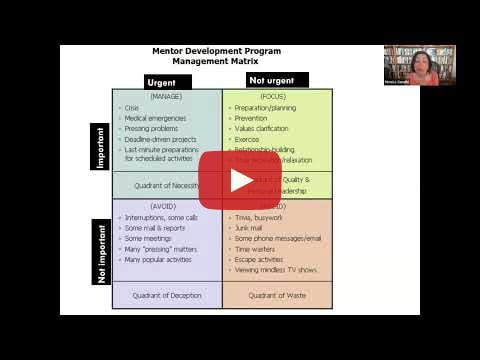
Mentoring Lecture Series
Time Management Techniques for Academicians
Presenter: Monica Gandhi, MD, MPH, University of California San Francisco (UCSF)
Job Postings
Infectious Diseases Research Job Openings
Texas A&M University - Postdoctoral Research Associate *New*
Washington University in St. Louis - Senior Research Technician - Pediatric Infectious Disease *New*
University of Virginia - Research Scientist, Division of Infectious Diseases
University of Pittsburgh - Associate/Assistant Professor of Epidemiology (Infectious Diseases)
Cincinnati Children's Hospital Medical Center - Professor, Division Director, Infectious Diseases
Emory University - Post Doctoral Fellow - Emory Vaccine Center
Baylor College of Medicine - Assistant Professor at the Vaccine Research Center
Visit the IDSA Career Center to browse other ID/HIV Medicine job postings.
Funding Opportunities
NIH Funding Opportunities Specific to COVID-19
This page contains a listing of active and expired funding opportunities.
Global Infectious Disease Research Administration Development Award for Low-and Middle-Income Country Institutions (G11 Clinical Trial Not Allowed) – Due March 13, 2024
The purpose of this notice of funding opportunity (NOFO) is to invite applications from research institutions in low- and middle-income countries (LMICs) to provide senior administrators from these institutions with advanced training in the management of NIH grants. The goal is to improve oversight of NIAID grant awards and compliance with NIH funding policies and Federal research funding requirements for NIAID-supported foreign institutions in LMICs.
Pulmonary Outcomes and Sequelae after Treatment-TB (POST-TB) (R01 Clinical Trial Optional) – Due May 7, 2024; Due September 7, 2024
The purpose of this Notice of Funding Opportunity (NOFO) is to support applications for epidemiological and observational research projects on the long-term cardiopulmonary sequelae following treatment for tuberculosis (TB). Investigators should propose additional testing and data collection in existing cohorts of adult and/or pediatric TB participants to better characterize and understand adverse outcomes and morbidity associated with TB disease post treatment in individuals with and without HIV infection.
RNA Delivery Technologies to Allow Specific Tissue
Target Homing (RNA-DASH)
– Due Friday, April 5
The purpose of this FOA is to support the development and/or pre-clinical studies of non-viral technologies to deliver RNA-based therapeutics into disease-relevant cells and tissues in vivo.
Centers for Disease Control and Prevention (CDC): Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases (ELC)
– Due Tuesday, April 30
The purpose of this FOA is to protect the public health and safety of the American people by enhancing the capacity of public health agencies to effectively detect, respond, prevent and control known an emerging (or re-emerging) infectious diseases.
NIAID Investigator Initiated Program Project Applications (P01 Clinical Trial Not Allowed) – Due June 8, 2024; 2025
This Funding Opportunity Announcement (FOA) invites submission of investigator-initiated Program Project (P01) applications. The proposed programs should address scientific areas relevant to the NIAID mission including: biology and pathogenesis of infectious microbes, including HIV; host-microbe interactions; mechanisms regulating immune system development and function across the lifespan, and in response to infectious pathogens; immune dysfunction resulting in allergy, asthma, autoimmunity, immunodeficiency, or transplant rejection; and translational research to develop vaccines, therapeutics, and diagnostics to prevent and treat infectious and immune-mediated diseases. Each P01 application submitted to this FOA must include at least two related, synergistic research projects that share a common central theme, focus, and/or overall objective; and an administrative core. A P01 may include
scientific cores, if needed for the proposed research.
International Research in Infectious Diseases (R01 Clinical Trial Not Allowed) – Due August 2, 2024; 2025
The purpose of this Funding Opportunity Announcement (FOA) is to support applications for high-priority, regionally relevant infectious diseases research by international investigators in resource-constrained countries. Applicant organizations must be headquartered in foreign (non-U.S.) resource-constrained countries (i.e. low-income economies, lower-middle-income economies, and upper-middle-income economies by World Bank Classification).
NIAID New Innovators Awards (DP2 Clinical Trial Not Allowed) – Due 30 days prior (LOI); 10/11/2024; 10/10/2025 (Full app)
The NIAID New Innovator Award supports postdoctoral and other candidates in non-independent positions or newly independent Early Stage Investigators of exceptional creativity who propose novel, original and insightful research concepts with the potential to produce a major impact, test scientific paradigms, or advance key concepts on broad, important problems in biomedical research of priority to NIAID. Applications proposing unexpected convergence of disciplines, new scientific directions, or the use of novel methodologies are encouraged. Applications from individuals with diverse backgrounds and in any topic relevant to the mission of NIAID are welcome.
Notice of Special Interest (NOSI): Halting Tuberculosis (TB) Transmission – Due January 07, 2026
The purpose of this Notice of Special Interest (NOSI) is to highlight NIAID’s interest in accepting applications that aim to understand the critical drivers of Tuberculosis (TB) transmission at the individual and population levels in high-burden settings. Applicants are encouraged to develop effective methods to measure rates of TB transmission that rely on an increased understanding of the biomedical basis of transmission and related risk factors and to develop and assess potential interventions, including low-cost and low-tech options, to prevent TB transmission.
Notice of Special Interest (NOSI): Complement in Fundamental Immunology – Due January 08, 2026
The main objective of this program is to support studies that accelerate our understanding of the roles of complement components and/or receptors in the initiation, magnitude, maintenance, and quality of immune responses involved in pathogenic infections, vaccination, post-infection sequelae, autoimmunity, allergy, or transplantation. The results of such studies will inform the development of vaccines or therapeutics that target complement components. The work to be encouraged includes studies of the roles of complement components (molecules and/or receptors) during immune responses.
Advancing Research Needed to Develop a Coccidioidomycosis (Valley fever) Vaccine –Due January 15, 2026
The purpose of this Notice of Special Interest (NOSI) is to highlight NIAID’s interest in supporting research in the areas outlined in the NIAID Strategic Plan For Research To Develop A Valley Fever Vaccine. The proposed research should have clear relevance to the strategic priorities defined in the strategic plan, which encompasses three major research areas: 1) address gaps in Coccidioides basic research to support the development of a vaccine; 2) develop tools and resources to support vaccine development; 3) develop and advance vaccines to prevent coccidioidomycosis.
Notice of Special Interest (NOSI): Using Targeted Degradation of Protein and non-Protein Targets for the Development of Novel Anti-Infectives – Due July 17, 2026
The purpose of this Notice of Special Interest (NOSI) is to invite applications for research on the use of targeted protein and nonprotein degradation (e.g., RNA) as it relates to the development of anti-infective strategies against viral, bacterial, parasitic, and fungal pathogens and/or their toxins (e.g., Lethal and Edema Toxins of Bacillus anthracis). Both novel monofunctional (e.g., Molecular Glues) and hetero-bi/tri-functional (e.g., PROTAC or PROTAC-like) strategies will be considered.
IDCRC Studies
Active Studies
Recruiting Volunteers
DMID Protocol 22-0019: A Phase 4 Study of a 3-Day vs. 7-Day Regimen of Doxycycline for the Treatment of Chlamydial Infection
Safety and Immunogenicity of CJCV2 With and Without ALFQ (DMID 19-0003)
- Pharmacokinetic Study of IV Aresunate to Treat Children With Severe Malaria (DMID 19-0007)
Fully Enrolled Studies
in Follow-up
Trial to Evaluate the Immunogenicity of Dose Reduction Strategies of the MVA-BN Monkeypox Vaccine
Safety, Tolerability, and Immunogenicity Study of Sm-p80 + GLA-SE (SchistoShield(R)) Vaccine in Healthy Adults
Meningococcal Serogroup ACYWX Conjugate Vaccine in Comparison With MenACWY-TT Conjugate Vaccine (DMID 20-0024)
Moderna’s mRNA-1273 vaccine, the KidCOVE Study (mRNA-1273-P204)
IDCRC Concept Quick Stats
ICP Status
Approved: 55
Administratively Not Supported: 29
Not Approved: 51
EWG Review: 2
EWG Liaisons: 0
EMT Concurrence: 0
Withdrawn: 15
Hold: 0
Moved to Active Study: 2
EWG Assignment
COVID: 92
Respiratory: 26
Emerging Infections: 13
Enteric Inf.: 7
Malaria and Tropical Dis.: 13
Sexually Transmitted Infections: 15
EVCP Status
EWG Review-In Process: 3
EMT Review: 2
Approved-moved to Prioritization: 5
Not Approved: 14
Approved-moved to Protocol development: 2
Active Study: 6
EMT Vote: 0
Study in Protocol Development: 5
Study Closed (LSLV Complete): 4
Other: 9
Communication Resources
Please submit IDCRC news to idcrc@emory.edu for inclusion in the monthly newsletter and IDCRC.org.