Profile: Jonathan Zenilman, MD

Read an interview with Jonathan Zenilman, MD, professor of medicine at Johns Hopkins University and IDCRC Clinical Operations Unit inaugural director.
“The IDCRC does more than COVID-19 and more than vaccines. We are interested in other areas, especially sexually transmitted infections, and we want to encourage concept development. Also, every member of the team is very invested in junior faculty development. This is an amazing track for developing the next generation of clinical trialists.” - Jonathan Zenilman, MD
Publications
NOTE: Please include the following citation in any publications resulting from direct or indirect IDCRC support:
"Supported by the Infectious Diseases Clinical Research Consortium through the National Institute for Allergy and Infectious Diseases of the National Institutes of Health, under award number UM1AI148684. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health."
View recent publications below:
- Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19
- Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection
- SARS-CoV-2 Serologic Assays in Control and Unknown Populations Demonstrate the Necessity of Virus Neutralization Testing
- Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19
- SARS-CoV-2 Infects Human Pluripotent Stem Cell-Derived Cardiomyocytes, Impairing Electrical and Mechanical Function
- Performance Analysis of the National Early Warning Score and Modified Early Warning Score in the Adaptive COVID-19 Treatment Trial Cohort
Training
IDCRC Mentee Profile: Christina Rostad, MD, Assistant Professor of Pediatrics, Division of Infectious Diseases, Emory University

Dr. Rostad’s research is focused on respiratory syncytial virus vaccine development, immune responses, and clinical vaccinology. Following the onset of the COVID-19 pandemic, she has additionally investigated serologic responses to SARS-CoV-2 in pediatric COVID-19 and its associated multisystem inflammatory syndrome.
Call for Applications: Advanced Course of Vaccinology (ADVAC 2022) – Due November 15
ADVAC is a two-week training program for decision-makers, including academia, industry, governmental and non-governmental agencies. The course aims to facilitate critical decision-making in vaccinology by providing participants with a comprehensive overview of the various aspects of vaccinology (immunology, vaccine development, clinical trials, regulatory processes, vaccine-specific issues including new vaccines, vaccination strategies and policies, program implementation, humanitarian emergencies, social, economic, political and ethical issues, financing, and communications).
Job Postings
View details on current job openings at the links below:
Mentoring Lecture Archive
Developing a Proposal: Laboratory Aspects of Clinical Protocol Development
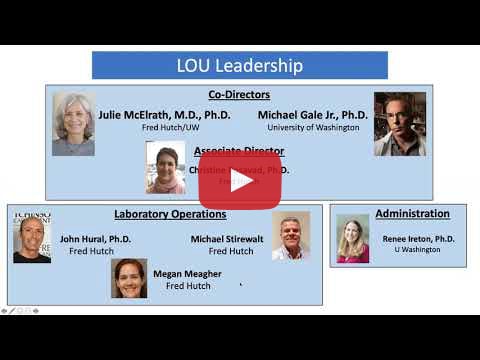
Presenter: Dr. Christine Posavad, Fred Hutchinson Cancer Research Center and University of Washington
IDCRC Studies
Active Studies
Recruiting Volunteers
Gritstone Second Generation COVID-19 Vaccine, CORAL Program
- Moderna’s mRNA-1273 vaccine, the KidCOVE Study
- SARS-CoV-2 Vaccines in Pregnancy and Postpartum, the MOMI-VAX Study
- Heterologous Prime Boost, Mix and Match Study
- Mucosal immunity against GC after 4CMenB Vaccination
Fully Enrolled Studies
in Follow-up
Moderna’s mRNA-1273 vaccine, The COVE Study™
Moderna’s mRNA-1273.351 Variant vaccine
AstraZeneca Study of AZD1222
The ENSEMBLE Study with Janssen’s Ad26.COV2.S Investigational Vaccine
Novavax Study of NVX-CoV2373
Regeneron’s 10933 and 10987 Monoclonal Antibodies, the REGN-COV2 Study
- Eli Lilly’s LY3819253 Antibody, the BLAZE-2 Study
- SARS-CoV-2/COVID-19 PREVALENCE STUDY, The COMPASS Study
IDCRC Concept Quick Stats
ICP Status
Approved: 25
Administratively Not Supported: 16
Revise and Resubmit: 7
Withdrawn: 7
Liaisons: 4
EWGs: 3
Not Approved: 26
EMT: 1
Other: 4
EWG Assignment
COVID: 69
Respiratory: 9
Sexually Transmitted Infections: 5
Malaria and Tropical Dis.: 3
Enteric Inf.: 2
Emerging Infections: 5
ECP Status
Protocol Development: 7
IDCRC Study Underway: 2
Submitted to ACTIV Mab Program per DMID: 1
Transitioned to CoVPN trial: 1
Transitioned to ACTT 2: 1
Funded by NIAID via other mechanism: 1
Pending: 7
Not approved: 0
In process: 4
On hold: 4
NOTE: Protocols Transitioned to IDCRC for Protocol Implementation: 5
In The News
UPMC and University of Pittsburgh participate in new COVID-19 vaccine study
Published July 26, 2021
UPMC and the University of Pittsburgh will be part of a nationwide study looking at the immune response to the COVID-19 vaccines in pregnant and postpartum women and their newborns.

5 new trials recruiting in ignored areas of women’s health
Published July 19, 2021
Throughout the pandemic, pregnant people have received mixed-messaging around whether it is safe for them to get a COVID-19 vaccine, as none have been clinically tested in this group. MOMI-VAX is a study sponsored and funded by the U.S. National Institute of Allergy and Infectious Diseases (NIAID) and being conducted by the Infectious Diseases Clinical Research Consortium (IDCRC). The trial is set to evaluate the immune responses generated by COVID-19 vaccines administered to pregnant or postpartum people.
COVID-19 Roundup: Moderna Neutralizes Delta Variant; FDA on Heart Inflammation Risk; and Vaccination During Pregnancy Studied
Published July 2, 2021
The COVID-19 vaccine from U.S. biotech firm Moderna is effective against the highly contagious Delta variant, Moderna stated in a news release, recounting results of recently completed studies that found the vaccine to have a neutralizing effect against most variants.
Communication Resources
Please submit IDCRC news to epthomp@emory.edu for inclusion in the monthly newsletter and IDCRC.org.