Second year in review | see how DiCE has progressed
The DiCE project has made significant strides in its second year (Oct 2023 - Oct.2024) in focusing on circular design for digital health devices. Using "10R strategies," the team created innovative concepts for redesigning smart pillboxes and identified environmental hotspots for endocutter improvements. User journeys for smart collection boxes have been refined, with pilots planned for 2025, and a digital application has been developed for real-time tracking of returned items.
In terms of technology and processes, DiCE achieved a remarkable 92% success rate in refurbishing Digital Display Labels (DDLs) across five cycles. Recycling tests on various devices have yielded valuable recommendations for design enhancements and more effective recycling processes. The project has also examined medical device regulatory frameworks and developed a draft reverse logistics plan for upcoming pilots.
Citizen engagement has been a key focus, with co-creation sessions and interviews conducted across Belgium, Spain, and Slovenia. These activities have provided insights into optimal collection locations and user preferences, which appear to be more age-related than geographically influenced. Small-scale pilots involving 12 families have tested motivational strategies, with preparations underway for larger confirmatory pilots.
DiCE has also made progress in performance assessment and exploitation, mapping value chains for various devices and selecting key circularity indicators. The project is developing a dynamic dashboard for real-time sustainability performance tracking. Additionally, a matrix of innovative circular business models has been created to facilitate the transition from linear to circular practices in the healthcare sector.
The project continues to address challenges in design, technology, regulation, and user engagement, while actively participating in conferences, workshops, and collaborations to share knowledge and engage stakeholders.
Second annual event | DiCE took on the Nordics
On 16 October 2024, in the fringe of the 6th Nordic Conference on Sustainable Healthcare conference, our expert team delivered an interactive annual event on the future of circular healthcare.
Gathering 30 professionals from across Europe, with the guidance of Thomas Mørch, Advisory Board Member and CEO of Zirq Solutions, our partners stepped on stage to share project results and foster discussions with the audience.
Webinar with CE-RISE project | How could the digital product passport be applied to digital health devices?
As the healthcare sector faces unique challenges due to stringent regulations and the need to ensure patient safety, this session, that took place on 29 October, focused on designing an ideal information-sharing system for digital health devices.
We discussed how data sharing can enhance circularity and improve value chains, with insights from real-life case studies and sector experts. Learn more in the recording below.

Building a strong consortium: DiCE experience
Building a strong consortium for DiCE is a rewarding experience. By focusing on our shared vision, enhancing communication and trust, leveraging diverse expertise, promoting generational collaboration, encouraging innovation, and committing to continuous improvement, we are well-equipped to tackle the challenges ahead.
We are continually inspired by the dedication and passion of our partners, and we are excited to see where this journey towards circularity will take us all. Together, we are not just participants in a project; we are pioneers shaping the future of circular digital health devices.
The importance of properly recycling medical WEEE: A call for circularity
Small medical WEEE, such as thermometers, glucose meters, and other portable devices like drug delivery systems or surgical endocutters, can easily come into direct contact with bodily fluids, such as blood or mucus. This contamination presents a legal and logistical hurdle for recycling because these items, once exposed to infectious materials, may no longer be classified as WEEE. Both European (Medical Device Regulations 178/2002 and 1223/2009) and Belgian regional governmental regulations, as well as the guidelines set by Recupel, dictate that once an item is considered a biohazard due to contamination, it must be treated differently and must therefore not be declared on the market as EEE.
These contaminated devices are not handled as electronic waste, but as medical waste, which requires specific treatment. In practice, the risk of exposure to hazardous biological material indeed means these items can no longer be directly processed in standard recycling streams without prior decontamination. This poses a significant problem for the circular economy and recycling goals, as potentially valuable materials that could be recovered from these devices may end up being incinerated or landfilled due to contamination concerns.
Brought to you by Lorenzo Glorie Senior Recycling Coordinator at RECUPEL
Student perspectives on recycling medical e-waste
As part of the DiCE project, numerous workshops were held in October and November 2023 to engage various groups in discussions about electronic waste recycling, including more than 50 students from the University of Maribor. These students represented two faculties: Faculty of Arts (Geography) and Faculty of Economics and Business. Their insights, collected during the workshops, offer a revealing glimpse into the perspectives of young people on the recycling of medical e-waste, an increasingly relevant issue in today’s world.
Brought to you by Špela Flegar, RDA Podravje – Maribor
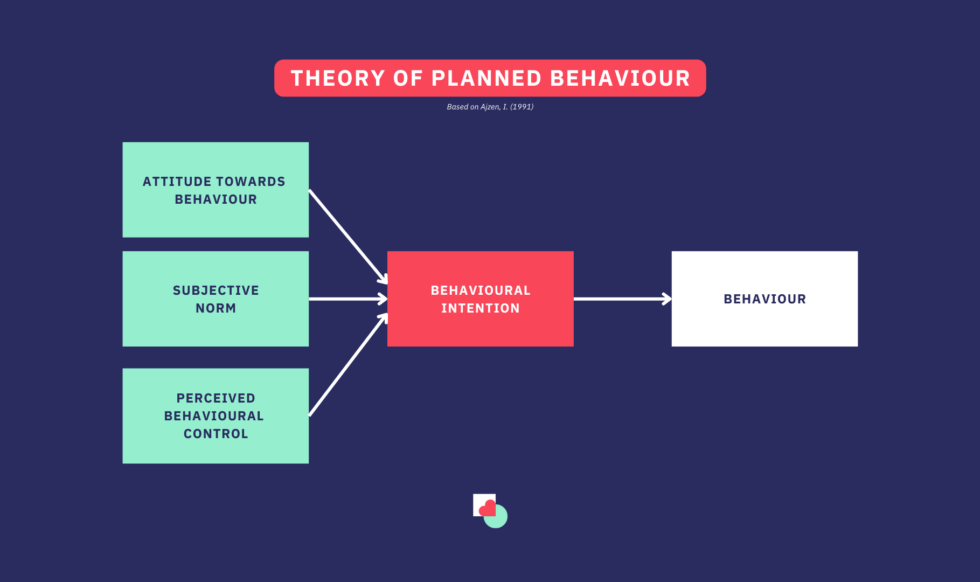
The psychology behind e-waste return
Understanding the motivations behind sustainable behaviour, particularly in the context of e-waste, requires insight into the psychological factors that drive individuals to act. One of the most successful theories in explaining motivations for sustainable behaviour is the Theory of Planned Behaviour (TPB). This well-established framework has been applied in various fields of research and has proven effective in explaining why people act the way they do (e.g., Puzzo & Prati, 2024). It consists of three main components: attitude, subjective norm, and perceived behavioural control (Ajzen, 1991). This raises the question: what exactly do these three components represent?
- Attitude refers to a person’s positive or negative evaluation of their behaviour. For instance, if someone believes that recycling electronic devices benefits the environment, they are likely to have a positive attitude towards participating in take-back programs.
- Subjective Norm involves the perceived social pressure to perform or avoid a behaviour. If individuals feel that their friends or community support recycling, they may feel encouraged to return their devices.
- Perceived Behavioural Control relates to a person’s belief in their ability to perform the behaviour. If someone believes they can easily access take-back programs and understand the recycling process, they are more likely to participate.
Together, these components influence the intention to act, with strong positive attitudes, supportive norms, and high perceived control increasing the likelihood of engaging in sustainable behaviours (Ajzen, 1991) like return electronic devices (Dixit & Badgaiyan, 2016).
Brought to you by Christiane Lehrer from Copenhagen Business School, Christian Meske and Hüseyin H. Keke from Ruhr-Universität Bochum
How can we more comprehensively assess the environmental impact of electronic healthcare devices?
A critical review recently conducted by Ghent University addresses this question. The study examined current scientific literature on the environmental impacts of EHDs using quantitative assessment methodologies (i.e., life cycle assessment (LCA) or footprint method). It identified a range of challenges and inconsistencies that hinder the ability to fully understand and mitigate the environmental burdens of these devices.
Brought to you by Erasmo Cadena Martinez, Naomi Muindi, Lieselot Boone, and Jo Dewulf from Ghent University alongside Wouter De Soete, and Kenneth Robertshaw from Johnson&Johnson MedTech
Unpacking the paradoxes of circular business models
The growing interest in circular business models has led numerous global sectors, including furniture (e.g. IKEA), healthcare (e.g. Philips), consumer electronics (e.g. Apple), and apparel (e.g. H&M), to implement circular principles into their product-service offerings and operations. The primary goal of adopting a circular business model is to minimise environmental impact while simultaneously creating financial value. For instance, Philips aims to generate 25% of their revenue from circular medical products in 2025.
However, despite their potential, circular business models are filled with paradoxes and trade-offs, that if not considered might undermine their intended benefits. As part of DiCE, we performed a large-scale interview study with 48 stakeholders in the healthcare industry and supplemented it with a literature review. From these studies, we would like to highlight three main paradoxes that illustrate the complexities and unintended impact of circular business models.
Brought to you by Camille Rønn from IT University of Copenhagen & Daniel Fürstenau from Freie Universität Berlin
The role of ethics in citizen participation
The active involvement of participants is essential in conducting pilot research projects to ensure that they respond to the real needs of citizens as well as meet their expectations and preferences. Their experiences, opinions and perspectives enrich the research process and form the core data of the research.
However, this involvement requires that the pilot undergoes an ethical review to ensure the well-being, confidentiality and rights of the participants.
This ethical review also benefits the research team, anticipating possible ethical dilemmas or carrying out modifications suggested by the ethics committee.
Brought to you by Mª Soledad Rojas and Elisa Sáez from the INTRAS Foundation
Sharing is caring
Do you know someone in your local network who would be interested in the outcomes of DiCE? Click here to download our leaflet in Slovenian, Flemish or Spanish. English speakers are no exception either, find your copy here! All interested parties are also invited to register to the DiCE network!


