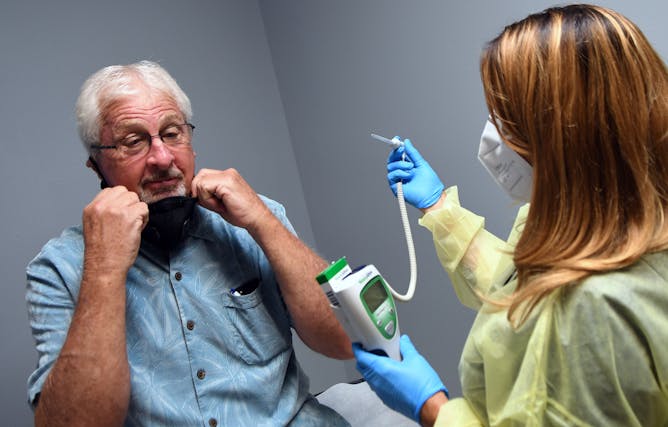
Tony Potts, a 69-year-old retiree, removes his face mask for a temperature check just before receiving his first injection in a phase 3 COVID-19 vaccine clinical trial sponsored by Moderna. Potts is one of 30,000 participants in the Moderna trial.
Paul Hennessy/NurPhoto via Getty ImageS
Christopher Robertson, Boston University; Jeremy Greene, Johns Hopkins University
The vaccines that will first be used to prevent the spread of COVID-19 will have gone through a special approval process with the FDA. But just what is this expedited process?
|
Economy + Business
|
-
Elizabeth C. Tippett, University of Oregon
Workers are increasingly making short videos of themselves on the job and posting them to TikTok, creating a new challenge for employers trying to police their behavior.
-
Andrew J. Hoffman, University of Michigan
Rather than pump money into a broken system, people like Jeff Bezos and Charles Koch could use their money to help fix it – by insulating politics from money.
|
|
Ethics + Religion
|
-
Ayanna Thompson, Arizona State University; Coen Heijes, University of Groningen
The annual Dec. 5 tradition sees performers don blackface and afro wigs. But a growing number of Dutch citizens believe it's time to wave goodbye to Black Pete.
|
|
Environment + Energy
|
-
Ernest Freeberg, University of Tennessee
A fast-moving equine flu cratered the US economy in the fall of 1872, showing all too clearly that horses were essential and deserved better treatment.
-
Caroline S. Chaboo, University of Nebraska-Lincoln
In the Amazon, beetles and flowering trees have developed a tight bond. Hundreds of beetle species thrive off of and pollinate blossoms, helping to maintain some of the highest biodiversity on Earth.
|
|
Arts + Culture
|
-
Lisa R. Pruitt, University of California, Davis
A stark divide in the response to the film suggests a deep disconnect between media elites and the rest of the country.
|
|
Politics/Election '20
|
-
Brian Glyn Williams, University of Massachusetts Dartmouth
Pulling out roughly half the U.S. troops in Afghanistan is part of an effort to find peace, but may unbalance a precarious stalemate.
|
|
Science + Technology
|
-
Sally Warner, Brandeis University
Some beaches in the world tend to consistently produce huge waves. Places like Nazaré Canyon in Portugal and Mavericks in California are famous for their waves because of the shape of the seafloor.
-
Matthew Woodruff, Emory University
The side effects of new SARS-CoV-2 vaccines are a result of immune system activation. While uncomfortable, they are both normal and expected. They are a sign that the vaccine is working.
|
|
Trending on site
|
-
Eugene Y. Chan, Purdue University
Here's how governments can get more people to follow COVID-19 guidelines.
-
Charles Bell, Illinois State University
Schools can consider virtual learning and other ways to reduce the negative impact of suspensions on student achievement.
-
Zachary Brewster, Wayne State University
It's long been known that Black patrons of bars and restaurants tend to get worse service than white customers. What's not been well understood is precisely why.
|
|