|
Many Americans hope that the advent of a vaccine will mean the beginning of the end of a pandemic that has cost almost 200,000 U.S. lives and trillions of dollars. But that won’t happen unless people have enough confidence in its safety to take it in sufficient numbers to achieve herd immunity. President Trump’s push to have a vaccine by Election Day has raised concerns that one will be released before it’s been proven safe and effective, prompting nine drugmakers to publicly pledge to avoid shortcuts in clinical trials.
Will it help?
Efthimios Parasidis, who has extensively studied vaccine policy, believes promises won’t be enough. The Ohio State University professor suggests two concrete steps lawmakers could take that would do a lot more to build public confidence in a vaccine.
Also today:
|
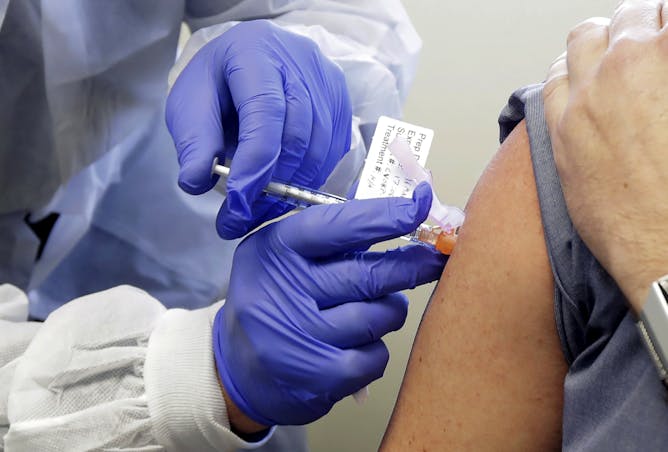
A patient receives a shot in a clinical trial for a COVID-19 vaccine.
AP Photo/Ted S. Warren
Efthimios Parasidis, The Ohio State University
Our best shot at ending the pandemic is by achieving herd immunity through widespread use of a vaccine. But that won't happen unless people believe it's safe.
|
Arts + Culture
|
-
Jenna Drenten, Loyola University Chicago
Increasingly outlandish gender reveal parties align perfectly with the values of an economy that's always scrolling for the next best thing.
|
|
Science + Technology
|
-
Douglas W. Jones, University of Iowa
Russian agents reportedly placed malware in U.S. voter registration systems in 2016 and are actively interfering in the 2020 election. Here's the state of election cybersecurity.
|
|
Politics/Election '20
|
-
Sher Jan Ahmadzai, University of Nebraska Omaha
In February, the US signed an accord with the Taliban to end the Afghanistan War. Now Taliban insurgents are meeting with the Afghan government – but peace remains an uncertain outcome.
-
Ofer Raban, University of Oregon
Those who say the Supreme Court's last term was a liberal success fail to understand that the types of decisions they see as victories are fleeting triumphs that will not endure.
|
|
Ethics + Religion
|
-
Hebah H. Farrag, University of Southern California – Dornsife College of Letters, Arts and Sciences; Ann Gleig, University of Central Florida
BLM has been accused of being 'Godless' and operating in a 'demonic realm.' But scholars of religion see a deep spirituality at work in the movement.
-
Joanne M. Pierce, College of the Holy Cross
Sept. 14 is the the Feast of the Holy Cross celebrated by many Catholics and some Christians. A scholar revisits the history of the cross, how it became a symbol of divine love, but also of violence.
|
|
Education
|
-
Samantha M. Brown, Colorado State University; Jenalee Doom, University of Denver
Kids become more vulnerable to maltreatment when their parents can't secure child care or housing or get jobs.
|
|
Trending on Site
|
-
Laurie Archbald-Pannone, University of Virginia
September is Alzheimer's Awareness Month and therefore a good time to talk about dementia. Alzheimer's is the most common dementia, but there are others to be aware of, a gerontologist explains.
-
Brett Goodin, New York University
Some 10,000 people are likely to give up their US passport this year, way above average. Are they fleeing COVID-19? Nasty politics? Taxes? None of the above, says an expert on American citizenship.
-
Karl Linden, University of Colorado Boulder
UV disinfection is a proven means of killing pathogens like the SARS-CoV-2 virus, but it's not risk-free.
|
|